2018年6月,细菌学研究国际顶级期刊《Cellular Microbiology》在线发表了南京农业大学动物医学院范红结教授团队在猪链球菌2型(SS2)致病机理研究领域所取得的突破性成果:The serine/threonine protein kinase of Streptococcus suis serotype 2 affects the ability of the pathogen to penetrate the blood brain barrier。
SS2是一种重要的人兽共患病原菌,除严重危害养猪业的发展外,于1998年、2005年分别在江苏、四川造成严重的公共卫生问题,引起重大的人员伤亡。SS2能引起人、猪发生脑膜脑炎,但机制不明。该团队在国家“973计划”、国家重点研发计划等项目的资助下,刘瑞、李唯一两位博士研究生利用转座子诱变技术,发现SS2丝氨酸/苏氨酸蛋白激酶系统(STKs/STPs)通过调控E3泛素连接酶(HECTD1),降解鼠脑微血管内皮细胞(bEnd.3)紧密连接蛋白claudin-5,增强血脑屏障的通透性。该研究首次阐明了SS2的STKs/STPs能降解bEnd.3的紧密连接蛋白,解析其降解机制,研究成果有助于进一步阐明SS2的致病机理,为新型药物靶标的发现及疫苗开发提供了理论指导。
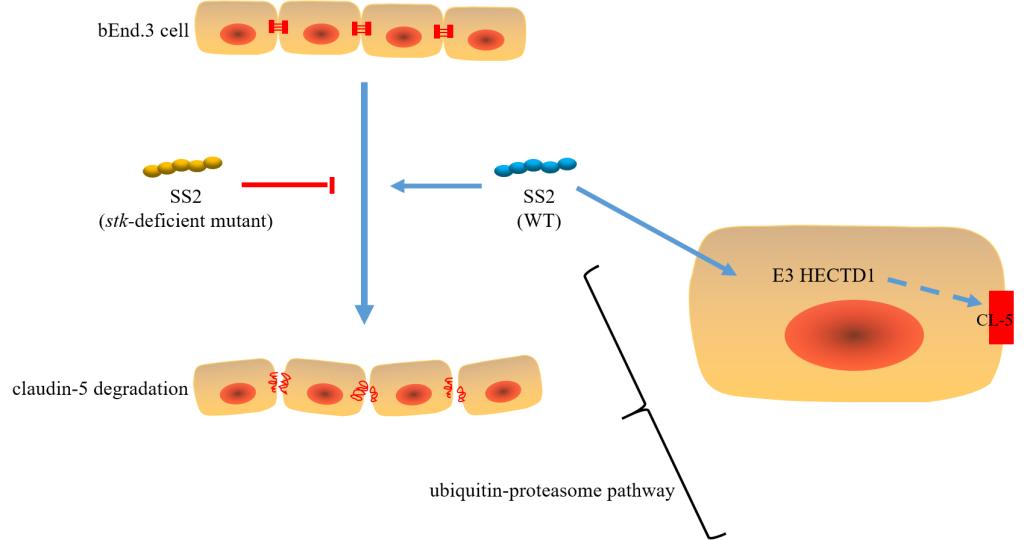
SS2的STKs/STPs降解bEnd.3紧密连接蛋白claudin-5的机制模式图
Abstract
Streptococcus suis serotype 2 (SS2) is a zoonotic agent that causes meningitis in humans and pigs. However, the mechanism whereby SS2 crosses the microvasculature endothelium of the brain is not understood. In this study, transposon (TnYLB-1) mutagenesis was used to identify virulence factors potentially associated with invasive ability in pathogenic SS2. A poorly invasive mutant was identified, and was found to contain a TnYLB-1 insertion in the serine/threonine kinase (stk) gene. Transwell chambers containing hBMECs were used to model the blood-brain barrier (BBB). We observed that the SS2 wild-type ZY05719 strain crossed the BBB model more readily than the mutant strain. Hence, we speculated that STK is associated with the ability of crossing blood-brain barrier in SS2. In vitro, compared with ZY05719, the ability of the stk-deficient strain (Δstk) to adhere to and invade both hBMECs and bEnd.3 cells, as well as to cross the BBB, was significantly attenuated. Immunocytochemistry using antibodies against claudin-5 in bEnd.3 cells showed that infection by ZY05719 disrupted BBB tight junction proteins to a greater extent than in infection by Δstk. The studies revealed that SS2 initially binds at or near intercellular junctions and crosses the BBB via paracellular traversal. Claudin-5 mRNA levels were indistinguishable in ZY05719- and Δstk-infected cells. This result indicated that the decrease of claudin-5 was maybe induced by protein degradation. Cells infected by ZY05719 exhibited higher ubiquitination levels than cells infected by Δstk. This result indicated that ubiquitination was involved in the degradation of claudin-5. Differential proteomic analysis showed that E3 ubiquitin protein ligase HECTD1 decreased by 1.5-fold in Δstk-infected bEnd.3 cells relative to ZY05719-infected cells. Together, the results suggested that STK may affect the expression of E3 ubiquitin ligase HECTD1 and subsequently increase the degradation of claudin-5, thus enabling SS2 to traverse the BBB.

