2018年5月底,国际病毒学顶级期刊《Journal of Virology》在线发表了南京农业大学动物医学学院姜平教授团队陈曦博士在猪繁殖与呼吸综合征病毒(PRRSV)领域所取得的突破性成果“Nsp1α of PRRSV strain BB0907 impairs the function of monocyte-derived dendritic cells via the release of soluble CD83”。
PRRSV是一种严重并持续危害全球养猪业的重要病原,致病机制尚不十分清楚。该团队在国家自然科学基金重点项目等资助下,博士研究生陈曦发现了PRRSV感染猪单核细胞衍生的树突细胞(MoDCs)通过诱导分泌CD83蛋白(sCD83),显著抑制MoDCs抗原提呈及其介导的T淋巴细胞增殖反应。首次阐明了PRRSV Nsp1α 的锌指结构域与激活CD83作用密切相关,是该病毒通过诱导MoDCs分泌sCD83抑制其免疫抑制的关键功能区域,进一步丰富了PRRSV免疫抑制的理论基础,对该病新型疫苗研究具有重要指导意义。
此前,该团队陈曦博士研究生发现并揭示了PRRSV N和Nsp10蛋白通过SP1和NF-κB信号通路诱导sCD83表达和分泌,发表于《Journal of Virology》。目前,该团队已经发表PRRSV相关研究论文110余篇,其中SCI论文41篇,研制的PRRSV活疫苗(R98株)实现了产业化生产,为我国该病防控发挥了重要作用。
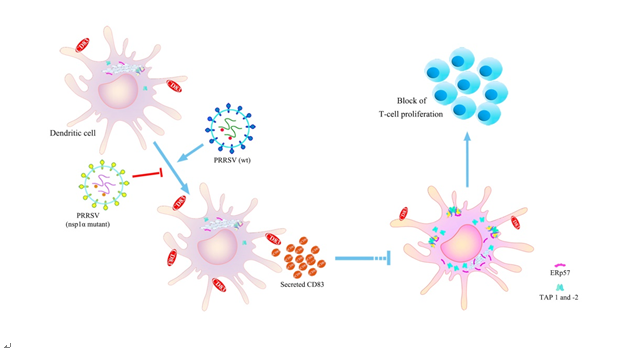
PRRSV通过sCD83介导免疫抑制的机制模式图
Journal of Virology 2018 May 23. pii: JVI.00366-18
Abstract:Porcine reproductive and respiratory syndrome virus (PRRSV), a virulent pathogen of swine, suppresses the innate immune response and induces persistent infection. One mechanism used by viruses to evade the immune system is to cripple the antigen processing machinery in monocyte-derived dendritic cells(MoDCs). In this study, we show that MoDCs infected by PRRSV express lower levels of the MHC-peptide complex proteins TAP1 and ERp57, are impaired in their ability to stimulate T cell proliferation, and increase their production of CD83.Neutralization of sCD83 removes the inhibitory effects of PRRSV on MoDCs. When MoDCs are incubated with exogenously added sCD83 protein, TAP1 and ERp57 expression decreases and T lymphocyte activation is impaired.PRRSV non-structural protein 1α (Nsp1α) enhances CD83 promoter activity. Mutations in the ZF domain of Nsp1α abolish its ability to activate the CD83 promoter. We generated recombinant PRRSVs with mutations in Nsp1α and the corresponding repaired PRRSVs. Viruses with Nsp1α mutations did not decrease levels of TAP1 and ERp57, impair the ability of MoDCs to stimulate T cell proliferation, or increase levels of sCD83. We show that the ZF domain of Nsp1α stimulates the secretion of CD83, which in turn inhibitsMoDC function. Our study provides new insights into the mechanisms of immune suppression by PRRSV.

