2017年6月,国际病毒学顶级期刊《Journal of Virology》在线发表了南京农业大学动物医学院姜平教授团队陈曦博士生在PRRSV领域所取得的突破性成果“The nucleocapsid protein and non-structural protein 10 of highly pathogenic porcine reproductive and respiratory syndrome virus enhance CD83 production via NF-κB and Sp1 signaling pathways”。
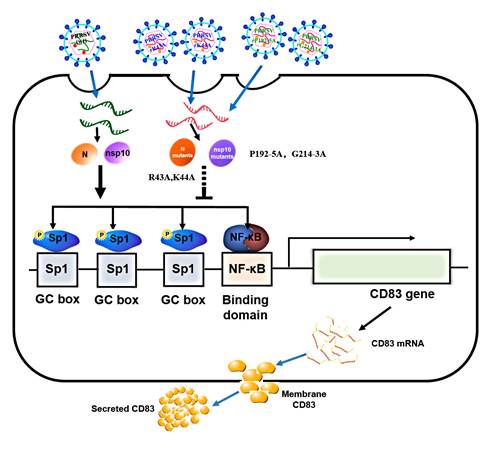
猪繁殖与呼吸综合征病毒(PRRSV)(俗称猪蓝耳病毒)是一种危害世界养猪业的重要病原,可引起感染猪群严重免疫抑制,但其免疫抑制尚不十分清楚。CD83是树突细胞(DCs)分泌蛋白,是DCs发挥其激发免疫应答作用不可或缺的功能性分子,但分泌性CD83(sCD83)具有强大的抑制混合淋巴细胞反应和DCs细胞介导的同种异体 T 淋巴细胞增殖反应。该团队在国家自然科学基金重点项目等资助下,致力于PRRSV免疫机制研究,博士研究生陈曦发现不同基因亚型PRRSV毒株感染猪单核细胞衍生的树突细胞(MoDCs)能够显著诱导CD83表达,探明该病毒编码蛋白N、Nsp1和Nsp10能够明显促进CD83表达。利用基因突变和返向遗传技术,构建成功一系列基因突变和回复突变重组病毒,阐明N蛋白第43-44及Nsp10第192-195和第214-213位置氨基酸在PRRSV感染刺激CD83 mRNA和蛋白分泌表达方面发挥关键作用。此外,在克隆分析CD83基因启动子序列基础上,利用重组病毒感染MoDC,进一步揭示了PRRSV通过激活SP1和 NF-κB信号通路诱导sCD83免疫抑制蛋白的表达和分泌。
该研究首次提出并且阐明了PRRSV上调CD83表达的分子机制,为PRRSV免疫抑制机制提供了新的见解,并对研究该病更有效的疫苗具有重要指导意义。此前,该团队在PRRSV流行病学、致病和免疫机制方面做了大量研究,发表研究论文110余篇,其中SCI论文39篇,并研制成功PRRSV活疫苗(R98株),获国家新兽药注册证书,为我国该病防控发挥了重要作用。
PRRSV上调CD83表达机制模式图
J Virol. 2017 Jun 28. pii: JVI.00986-17. doi: 10.1128/JVI.00986-17.
The nucleocapsid protein and non-structural protein 10 of highly pathogenic porcine reproductive and respiratory syndrome virus enhance CD83 production via NF-κBand Sp1 signaling pathways
Abstract:Porcine reproductive and respiratory syndrome, caused by the virus of the same name (PRRSV) is a panzootic disease, and one of the most economically costly to the swine industry. A key aspect of PRRSV virulence is that the virus suppresses the innate immune response and induces persistent infection, although the underlying mechanisms are not well understood. The dendritic cell (DC) marker CD83 belongs to the immunoglobulin superfamily and is associated with DC activation and immunosuppression of T cell proliferation when expressed as soluble CD83 (sCD83). In this study we show that PRRSV infection strongly stimulates CD83 expression in porcine monocyte-derived DCs (MoDCs), and that the nucleocapsid (N) protein and non-structural protein 10 (nsp10) of PRRSV enhance CD83 promoter activity via the NF-κB and Sp1 signaling pathways. Amino acid substitution mutants R43A and K44A of N protein suppress the N protein-mediated increase of CD83 promoter activity. Similarly, P192-5A and G214-3A of nsp10 abolish the nsp10-mediated induction of the CD83 promoter. Using reverse genetics, four mutant viruses (rR43A, rK44A, rP192-5A and rG214-3A) and four revertants (rR43A(R), rK44A(R), rP192-5A(R) and rG214-3A(R)) were generated. Decreased induction of CD83 in MoDCs was observed after infection by mutants rR43A, rK44A, rP192-5A and rG214-3A, in contrast to the results obtained using rR43A(R), rK44A(R), rP192-5A(R) and rG214-3A(R). These findings suggest that PRRSV N protein and nsp10 play important roles in modulating CD83 signaling, and shed light on the mechanism by which PRRSV modulates host immunity.

